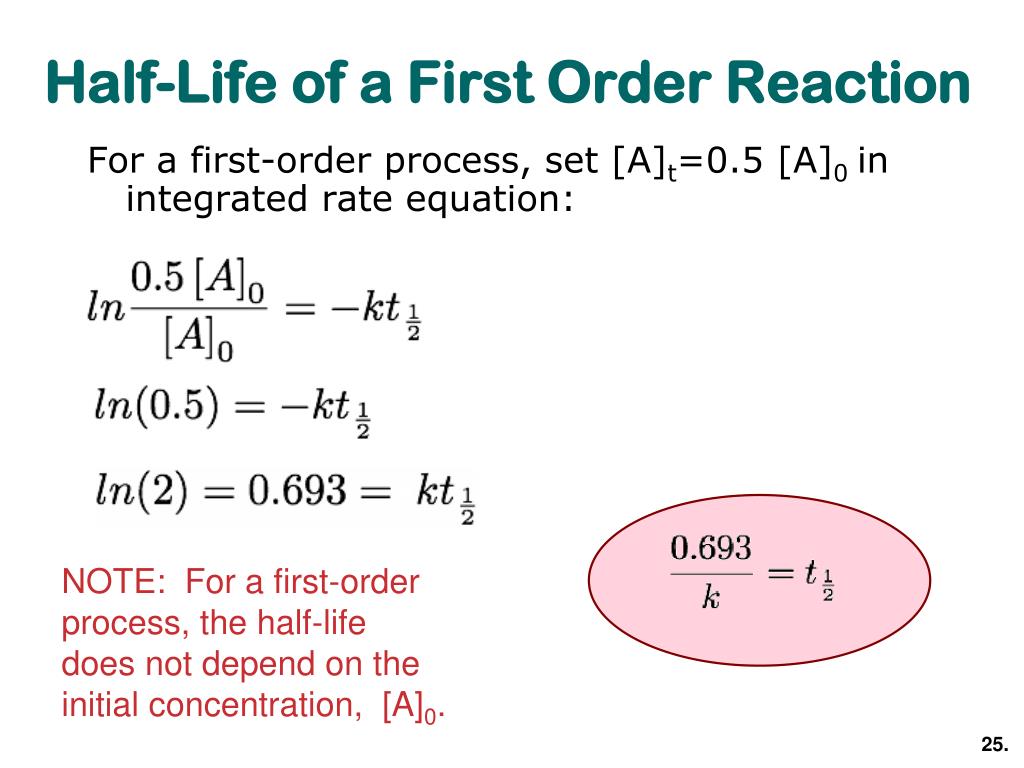
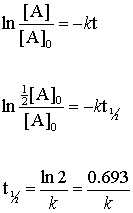half life formula for first order reaction
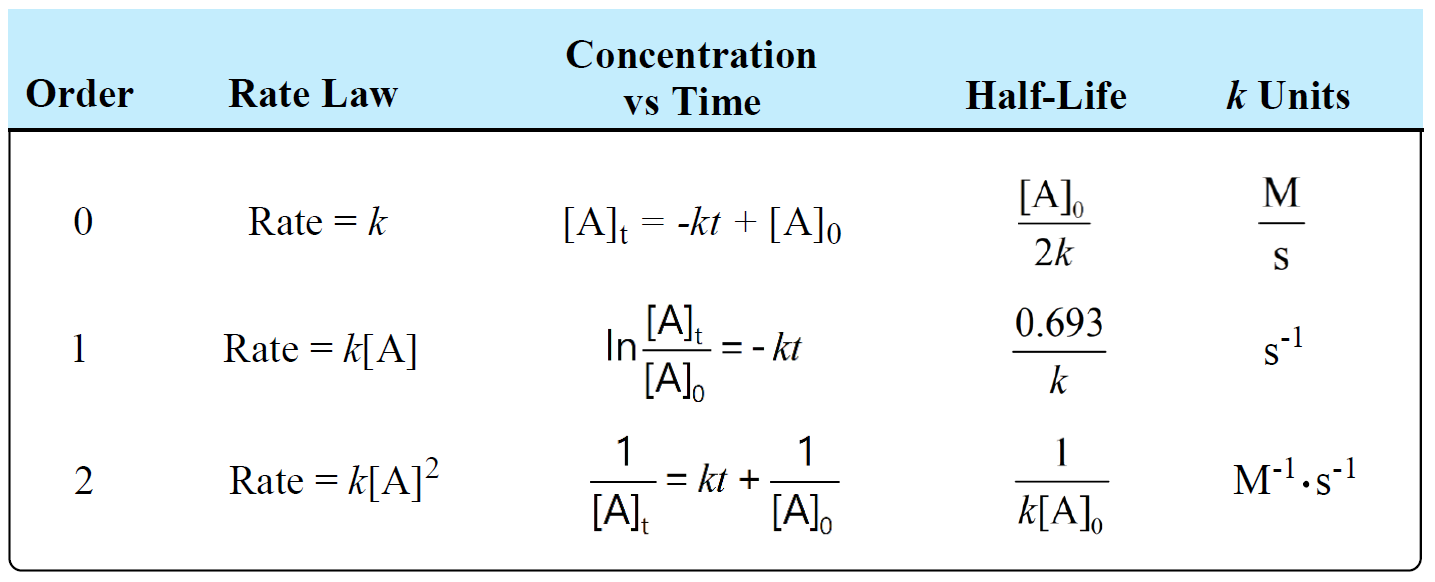
The unit of half-life equation for first order reaction is also second 3. The half-life of a first-order reaction is given as t 12 0693k.
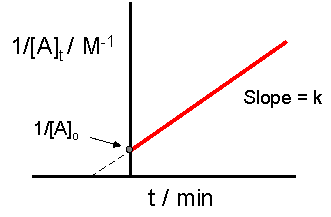
2 8 Second Order Reactions Chemistry Libretexts
If we know the integrated rate laws we can determine the half.
. Added Dec 9 2011 by ebola3 in Chemistry. Half-life formula and unit for. The half-life of a first-order reaction is a constant that is related to the rate constant for the reaction.
Using the half-life equation derived from the concentration-time equation as shown in example 1 we can solve for the initial concentration of reactant. The half-life of a second-order reaction is given by the formula 1kR 0. Substituting these terms into the rearranged integrated rate law and simplifying yields the equation for half-life.
How to calculate Half Life period of first order reaction using this online calculator. So here is your half-life for a first order reaction. The rate constant k for the reaction or enough information to determine it.
This widget calculates the half life of a reactant in a. The First Order Half-Life calculator computes the first order half-life based on the temperature dependent rate constant. What is the formula for half-life of a drug.
In some cases we need to know the initial. What is the expression for Half-Life of a First Order ReactionHere I derive it from the integrated rate lawThe answer is t ln 2 kAsk me questions. The order of the reaction or enough information to determine it.
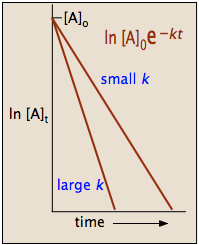
The half-life t12 is the. We can see that the half-life of a first-order reaction is inversely. Emulsions Where The half.
The half-life of a first-order reaction is given as t 12 0693k. परथम कट क अभकरयए First Order Reaction समकलत वग समकरण Integrated Rate. For the first-order reaction the half-life is defined as t12 0693k And for the second-order reaction the formula for the half-life of the reaction is given by 1k R 0 Where t12 is the half.
Where The half-life of a reaction is referred to as t 12. The half-life formula used to calculate the first-order reaction is t₁₂ 0693k. Half life formula of nth order reaction is t 1 2 2 n 1 1 A 0 n 1 n 1 k Problem practice If the half life period for a first order reaction in A is 2 minutes How long will it take.
The half-life is the time required for a quantity to fall to half its initial value as measured at the beginning of the time period. The half-life of a second-order reaction is given by the formula 1kR 0. T_ 12 frac 1.
To use this online calculator for Half Life period of first order reaction enter Rate Constant K h and hit. So our half-life is equal to let me rewrite this here so our half-life t 12 is equal to 693 divided by k where k is our rate constant. Half Life Calculator first order reaction input the equations calculated rate constant.

Rate Equation And Order Of Reaction
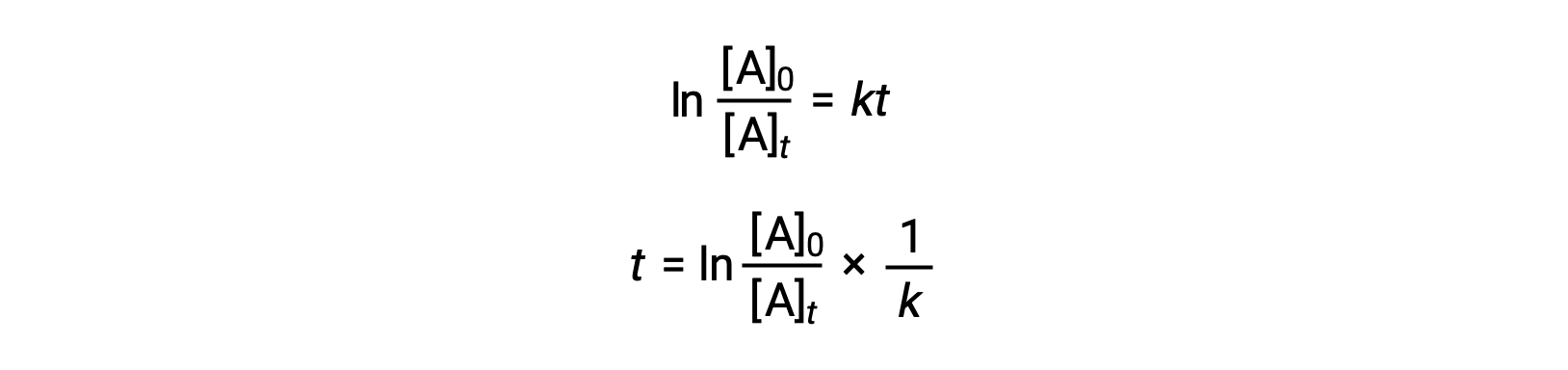
Jove Peer Reviewed Scientific Video Journal Methods And Protocols

How To Calculate Half Life Of A First Order Reaction Chemistry Study Com
Ppt Chemical Kinetics Chapter 13 Powerpoint Presentation Free Download Id 4450066
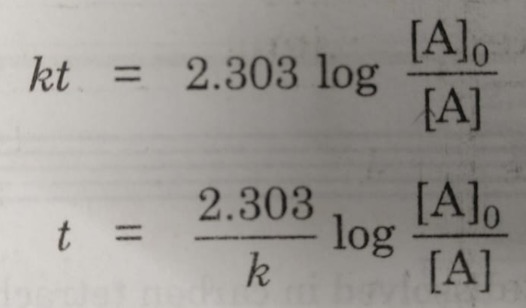
Half Life Period Of A Reaction Chemical Kinetics Chemistry Class 12

Half Life Of A First Order Reaction Video Khan Academy

First Order Reaction Definition Examples And Equations
A Derive The General Form Of The Expression For The Half Life Of A First Order Reaction Sarthaks Econnect Largest Online Education Community
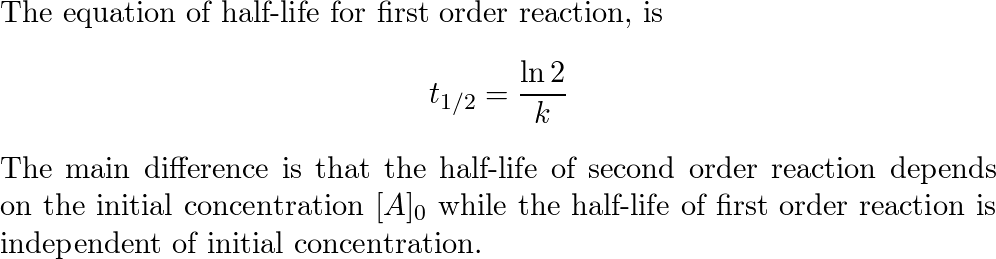
Write The Equations Relating The Half Life Of A Second Order Quizlet
Units Of Rate Constant K Chemistry Steps

Physical Chemistry Formula For Rate Constant For The First Order Reaction Chemistry Stack Exchange
Concentration Time Relationships Integrated Rate Laws Introductory Chemistry
17 2 Reaction Rates Typically Change With Time Chemistry Libretexts

How To Calculate Half Life Of A Second Order Reaction Chemistry Study Com
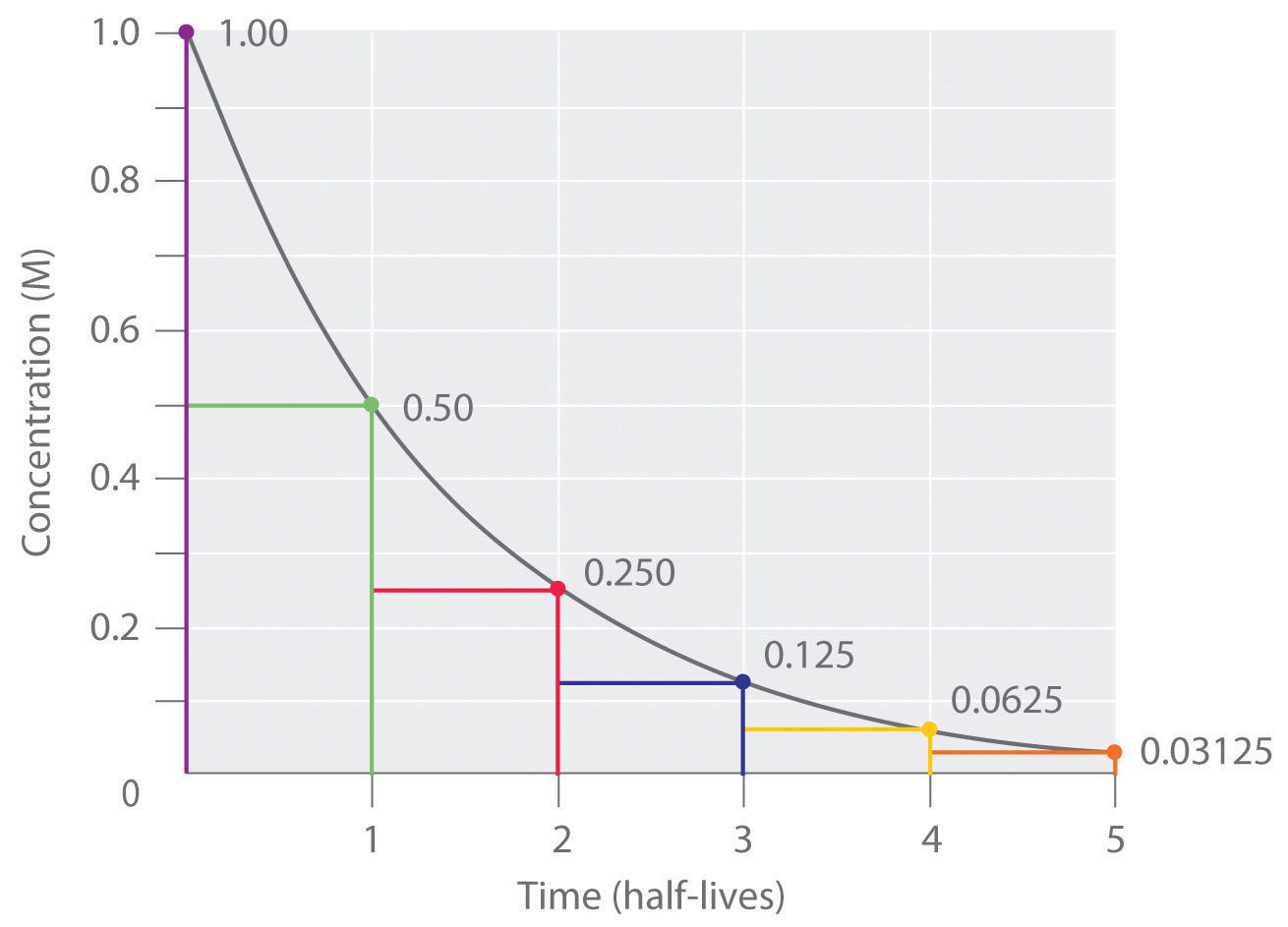
Half Lives And Radioactive Decay Kinetics
I What Is Meant By Half Life Period Of A Reaction Ii By Deriving The Equation For T1 2 Of First Order Reaction Sarthaks Econnect Largest Online Education Community